Testing for COVID-19 is very much on people’s minds at the moment. I had an email from someone who works in a virology lab, who is doing a lot of testing for the disease at the moment, and wants to remain anonymous. I thought it was interesting; I don’t mean to suggest it’s the whole story, but I thought I’d pass it on.
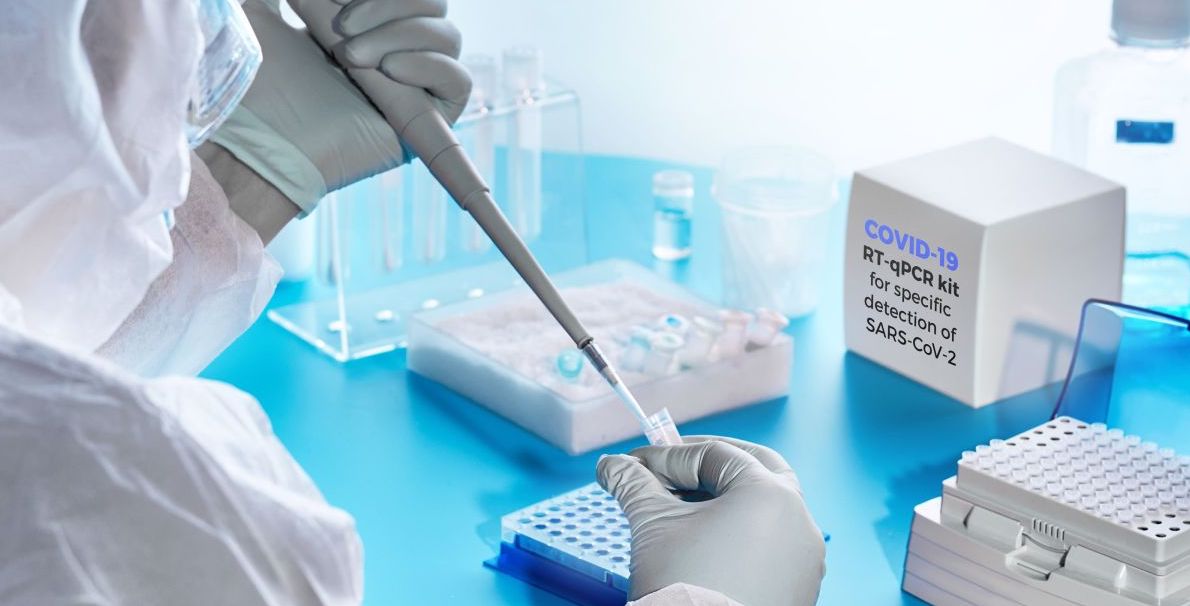
Her lab does polymerase chain reaction (PCR) tests, the current standard. They work by finding a unique length of viral RNA, and multiplying it lots and lots. To extract the RNA, you need a specific chemical, called a reagent.
The pre-PCR extraction machine her lab uses to extract the RNA for the PCR process is made by the Swiss firm Roche. “It can test 96 samples in 90 minutes,” she says, “which is great for testing large numbers quickly.” Lots of other labs use it too.
But the reagents come in a specific kit that only Roche makes, and — unsurprisingly — Roche is unable to meet demand at the moment. We’ve heard that UK companies could make the required chemicals, but that’s not the whole story. “The chemical components of the reagent may well be very easy to make in the UK,” she says. “But the reagent has to be in a Roche kit for a Roche machine to take it.” It’s like saying you could use Hewlett Packard toner in a Canon printer — maybe you could, in theory (the inks might be the same), but the cartridge wouldn’t fit, so you can’t.
While her lab is waiting for more Roche supplies, they’re using other testing platforms, which can only do 24 or 48 samples at a time. “Some other labs are moving to slower platforms, like Qiagen,” she says, “which can do 96 samples [at a time], but take about 5 hours rather than 90 minutes.”
She speculates that, since Qiagen is a German company and German labs use a mix of Roche and Qiagen machines, that might explain why Germany is further ahead on testing.
I’m not suggesting this explains the whole problem, or that it means the government is blameless. If the UK virology industry is hugely over-reliant on Roche machines for testing, then I guess you could say that’s a foreseeable problem — but “the virology industry is small, and in normal non-pandemic times there is always a reason behind scientists picking the machines they pick, and doing things the way they do,” says my correspondent. This will surely be the biggest boom in virology-test demand in history by several orders of magnitude; I doubt any firm could keep up with it.
No doubt there are more things the government could be doing, and it’s important to keep pressing them for more openness. At the moment, though, I’m most interested in rolling out serology tests that could tell us who’s already had the disease. Then we can start answering the really tough questions.









Join the discussion
Join like minded readers that support our journalism by becoming a paid subscriber
To join the discussion in the comments, become a paid subscriber.
Join like minded readers that support our journalism, read unlimited articles and enjoy other subscriber-only benefits.
Subscribe