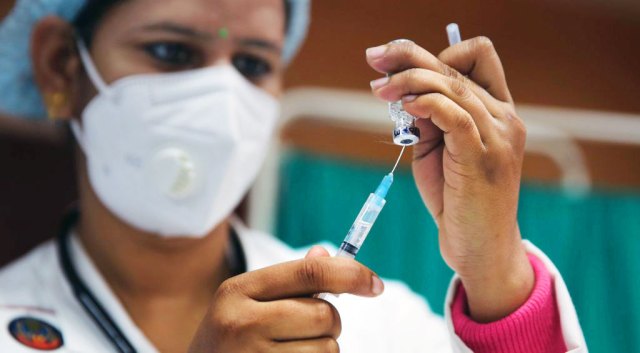
Only one factory in India is allowed to produce the Oxford-AstraZeneca vaccine. Credit: Himanshu Vyas/Hindustan Times via Getty Images

A gargantuan yet widely unreported struggle is taking place in Geneva that could affect how quickly billions of people are vaccinated against Covid-19. It’s a struggle that will also affect how soon effective treatments will be available. Depending on how it is resolved, it could change the landscape of medical innovation permanently — and take decisions about which diseases are prioritised out of the hands of the pharmaceutical industry.
Last October, two members of the World Trade Organisation (WTO), India and South Africa, proposed a waiver that would allow countries to choose not to enforce certain intellectual property (IP) rights relating to tests, treatments, equipment and vaccines for Covid-19. If approved, this would mean that for the duration of the pandemic, a country could locally manufacture a vaccine, ventilator or Covid-19 test while they were still under patent elsewhere, boosting supplies and getting these technologies to those who need them faster. At the moment, with rare exceptions, manufacturers must be licensed by the patent-holder for the duration of the patent, which is typically 20 years.
The lobbying has been intense over the last few months, but those countries that have already declared their positions on the waiver have done so along predictable lines. The poorer states of the Global South, which tend to be at the back of the queue for vaccines and everything else, have aligned themselves with India and South Africa; the wealthy Global North, where the companies holding the patents also tend to be headquartered, is broadly opposed. As Mira Johri, professor of global health at the University of Montreal, puts it: “The map of vaccine roll-outs looks a lot like the map of who is opposed to the WTO waiver.”
The pandemic has highlighted the fact that demand and supply don’t necessarily go together, in turn raising the question of whether our system for producing new medicines — driven as it is by commercial incentives — is fit for purpose. Indeed, its fragility was highlighted only last night, when it was revealed that the EU is threatening to block exports of the Belgian-produced Pfizer jab – of which the UK is expecting almost 3.5 million doses.
More broadly, it’s a simple fact that rich countries are serving themselves first – but in practising such “vaccine nationalism” they are doing the world a disservice. Leaving poorer countries without vaccine protection could trigger a number of undesirable consequences for every nation — from disrupting global supply chains, including of the vaccine itself, to potentially driving the emergence of new variants of the virus. As the WHO’s director-general Tedros Adhanom Ghebreyesus said earlier this month: “Vaccine nationalism hurts us all and is self-defeating.”
Since the pandemic first hit, it has become clear that well-meaning initiatives like COVAX — which brings together governments, global health organisations and the private sector to try to ensure equitable global access to Covid-19 vaccines — are not enough to counter vaccine nationalism. (Though last week’s announcement that the US will join COVAX, having stayed out under the Trump administration, could help.) Of the 7 billion vaccine doses whose advance purchase has been confirmed, more than half (4.2 billion), have gone to high-income countries, whose combined population is around 1 billion, out of a global total of nearly 8 billion. Only around 1.5 billion doses have gone to middle-income countries (combined population around 6 billion) and just 270 million doses have gone to low-income countries. COVAX, meanwhile, has secured around 1 billion doses.
The waiver proposed by India and South Africa is an attempt to achieve equitable access in a different way — by wresting control of lifesaving technologies from the patent-holders, and opening up their manufacture to generic producers sooner than those patents allow. Those who support it argue that it is, if nothing else, a wise insurance policy in a pandemic. For instance, the vaccine-producing colossus, the Serum Institute of India (SII), is the only licensed Indian supplier of the Oxford-AstraZeneca vaccine, though the country has many vaccine manufacturers.
But because India has a bad local epidemic, its government has decided that SII must meet emergency domestic needs before it ships to COVAX, which means that those shipments are likely to be delayed until the spring. And on 21 January, a fire broke out there — though SII claimed this had not affected vaccine production. “Is it sensible to have one Indian company producing so much of your vaccine?” asks Yuanqiong Hu, a senior legal and policy advisor for the international NGO Médécins Sans Frontières (MSF) in Geneva.
MSF, which is supporting the Covid-19 response in more than 70 countries, also claims that patent-holders are putting profits before lives, giving as an example South Africa’s recent difficulties in obtaining proprietary chemicals used in Covid-19 tests, partly due to patent-holders not wanting to release formulas. There is an unfortunate precedent for this. At the beginning of South Africa’s HIV/AIDS epidemic, President Nelson Mandela’s government took steps to reduce the cost of expensive new antiretroviral drugs, including issuing compulsory licences to allow local production.
Some 40 drug companies sued. The Global South, led by India, Brazil and South Africa itself, protested, and the result was the 2001 Doha “Declaration on the TRIPS Agreement and Public Health”, which allowed governments to temporarily waive their obligations under the Trade-Related Aspects of Intellectual Property Rights agreement.
India and South Africa are seeking a similar waiver now, but the pharmaceutical industry says it isn’t the answer. In a recent New York Times op-ed, the director general of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) wrote that the record speed with which Covid-19 vaccines have been produced was only possible because patents protect investment in high-risk technology, allowing developers to recoup their costs. Yes, public money had been poured into the Covid-19 vaccine effort, but that funding, Thomas Cueni wrote, “principally helped reduce risk and accelerate production timelines — the research and development were still driven by scientists in the private sector”.
We have to take his word for it, because companies do not disclose either how much they invest or how much they earn. As Johri points out: “It is difficult to know if a balance is being struck.” But it is true that for decades before Covid-19, companies were deserting the vaccine field, an indication that they at least judged the profit margins to be too slim. For Andrew Lo, an economist at MIT, a waiver would have a chilling effect on research and development investment which would make itself felt in the next pandemic in a dearth of medicines and equipment.
In his op-ed, Cueni described the South African lawsuit over HIV/AIDS drugs as a “terrible misjudgement” and claimed that the industry had learned from its mistakes. “The current situation is not parallel,” he wrote. For one thing, the IFPMA is a partner of COVAX, along with generic producers and the WHO. But while it insists that it understands its responsibility to come up with an exceptional response to the exceptional circumstances of the pandemic, the IFPMA says that no solution can come at the price of safety and quality.
Scaling up manufacturing capacity is a different order of challenge for vaccines than for most antiretroviral drugs, argues Guilherme Cintra, the association’s director of innovation policy, because vaccines are given to healthy people and the quality checks are correspondingly more stringent. If the developer loses oversight of supply and production lines too soon, quality could be compromised and — against a backdrop of high levels of vaccine hesitancy — public trust undermined at a critical juncture in the vaccine rollout.
There is one thing that everyone agrees on: the proposed waiver is a blunt instrument. Even if the TRIPS Council votes for it at its next meeting in March (rather than voting against it, or failing to reach a consensus as it did in December), like Doha it will need to be translated into national legislation before it can be implemented, and that could take months.
Its true value might lie elsewhere, however. “Sometimes things are most effective as a bargaining tactic,” says Johri. “If there is a real threat of a waiver of IP rights, companies tend to share more or to reduce prices quickly.” Though the lawsuit against South Africa was eventually dropped and Doha’s provisions were eventually watered down, UNAIDS estimates that the proportion of patients able to access antiretroviral drugs increased from 3% in 2002 to 47% in 2010, thanks largely to price reductions that wouldn’t have happened without Doha.
Doha achieved its goal indirectly, then, but Hu says the lives of millions more HIV/AIDS patients could have been saved had action been taken earlier. And there are other diseases still exacting a terrible toll, or threatening to do so again — tuberculosis and Ebola, for example — for which the same is true. “We need a long-term solution,” she says.
Even the IPFMA acknowledges that there is room for debate over the right balance of responsibility and reward between public and private sectors. Andrew Lo agrees, but for him the waiver on the table in Geneva remains a distraction. “The real issue is that drug pricing and access is not just about economics, there’s an ethical dimension as well,” he says. “If we as a society believe that everyone should be vaccinated, regardless of cost, then we should simply use government funds to acquire the rights to the technology at market prices and then vaccinate everyone for free.”
But should we even let the pharmaceutical industry decide which vaccines or treatments are developed in the first place? That’s the reason why, in general, the diseases of the rich world are better served than those of the poor world. Other ways are possible, without sacrificing incentives to innovate. A well-funded global health organisation could offer a prize for the vaccine or treatment most likely to reduce the global burden of a given disease, for example. On this model, the organisation would have some ownership of the winning product and be better placed to ensure its equitable distribution.
This pandemic has revealed two truths that sit uncomfortably together: first, a lot of medical innovation is driven by publicly funded academia (think of the Oxford in Oxford-AstraZeneca); second, governments protect one industry — pharmaceuticals — at the expense of all the others, which will take years if not decades to recover from Covid-19. These realisations indicate that it’s time for a reset. The TRIPS Council could at least set the ball rolling. But the whole of society needs to join the debate.








Join the discussion
Join like minded readers that support our journalism by becoming a paid subscriber
To join the discussion in the comments, become a paid subscriber.
Join like minded readers that support our journalism, read unlimited articles and enjoy other subscriber-only benefits.
Subscribe